What is the most important information I should know about PADCEV?
PADCEV may cause serious side effects, including:
Skin reactions.
Skin reactions including severe skin reactions have happened in people treated with PADCEV and may be more common when PADCEV is given with pembrolizumab. In some cases, these severe skin reactions have caused death. Most severe skin reactions occurred during the first cycle of treatment but may happen later. Your healthcare provider will monitor you, may stop your treatment with PADCEV completely or for a period of time (temporarily), may change your dose, and may prescribe medicines if you get skin reactions. Tell your healthcare provider right away if you develop any of these signs of a new or worsening skin reaction:
•
Target lesions (skin reactions that look like rings)
•
Rash or itching that continues to get worse
•
Blistering or peeling of the skin
•
Painful sores or ulcers in mouth or nose, throat, or genital area
•
Fever or flu-like symptoms
•
Swollen lymph nodes
See “What are the possible side effects of PADCEV?”
for more information about side effects.

Before receiving PADCEV, tell your healthcare provider about all
of your medical conditions, including if you:
•
Are currently experiencing numbness or tingling in your hands or feet.
•
Have a history of high blood sugar or diabetes.
•
Have liver problems.
•
Are pregnant or plan to become pregnant. PADCEV can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with PADCEV.
•
Are breastfeeding or plan to breastfeed. It is not known if PADCEV passes into your breast milk. Do not breastfeed during treatment and for 3 weeks after the last dose of PADCEV.

Females who are able to become pregnant:
•
Your healthcare provider should do a pregnancy test before you start treatment with PADCEV.
•
You should use an effective method of birth control during your treatment and for at least 2 months after the last dose of PADCEV.

Males with a female sexual partner who is able to become pregnant:
•
If your female partner is pregnant, PADCEV can harm the unborn baby.
•
You should use an effective method of birth control during your treatment and for at least 4 months after the last dose of PADCEV.

Tell your healthcare provider about all the medicines you take,
including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking PADCEV with certain other medicines may cause side effects.
What are the possible side effects of PADCEV?
PADCEV may cause serious side effects, including:

Skin Reactions.
See
“What is the most important information I should know about PADCEV?”
Skin Reactions. See “What is the most important information I should know about PADCEV?”

High blood sugar (hyperglycemia).
An increase in blood sugar is common during treatment with PADCEV. Severe high blood sugar, a serious condition called diabetic ketoacidosis (DKA), and death have happened in people with and without diabetes treated with PADCEV. Tell your healthcare provider right away if you have any symptoms of high blood sugar, including: frequent urination, increased thirst, blurred vision, confusion, it becomes harder to control your blood sugar, drowsiness, loss of appetite, fruity smell on your breath, nausea, vomiting, or stomach pain.
High blood sugar (hyperglycemia).
An increase in blood sugar is common during treatment with PADCEV. Severe high blood sugar, a serious condition called diabetic ketoacidosis, and death have happened in people with and without diabetes treated with PADCEV. Tell your healthcare provider right away if you get any symptoms of high blood sugar, including: frequent urination, increased thirst, blurred vision, confusion, it becomes harder to control your blood sugar, drowsiness, loss of appetite, fruity smell on your breath, nausea, vomiting, or stomach pain.

Lung problems.
PADCEV may cause severe or life-threatening inflammation of the lungs that can lead to death. These severe problems may happen more often when PADCEV is given in combination with pembrolizumab. Tell your healthcare provider right away if you get new or worsening symptoms, including trouble breathing, shortness of breath, or cough.

Nerve problems.
Nerve problems, called peripheral neuropathy, are common during treatment with PADCEV and can sometimes be severe. Nerve problems may happen more often when PADCEV is given in combination with pembrolizumab. Tell your healthcare provider right away if you get new or worsening numbness or tingling in your hands or feet or muscle weakness.

Eye problems. Certain eye problems are common during treatment with PADCEV. Tell your healthcare provider right away if you have dry eyes, increased tearing, blurred vision, or any vision changes. You may use artificial tear substitutes to help prevent or treat dry eyes.
Eye problems. Certain eye problems are common during treatment with PADCEV. Tell your healthcare provider right away if you get dry eyes, increased tearing, blurred vision, or any vision changes. You may use artificial tear substitutes to help prevent or treat dry eyes.

Leakage of PADCEV out of your vein into the tissues around your infusion site (extravasation).
If PADCEV leaks from the injection site or the vein into the nearby skin and tissues, it could cause an infusion site reaction. These reactions can happen right after you receive an infusion, but sometimes may happen days after your infusion. Tell your healthcare provider or get medical help right away if you notice any redness, swelling, itching, blister, peeling skin or discomfort at the infusion site.
Leakage of PADCEV out of your vein into the tissues around your infusion site (extravasation).
If PADCEV leaks from the injection site or the vein into the nearby skin and tissues, it could cause an infusion site reaction. These reactions can happen right after you receive an infusion, but sometimes may happen days after your infusion. Tell your healthcare provider or get medical help right away if you notice any redness, swelling, itching, blistering, peeling skin or discomfort at the infusion site.
Your healthcare provider may decrease your dose of PADCEV, or temporarily or completely stop your treatment with PADCEV if you have severe side effects.
Your healthcare provider may decrease your dose of PADCEV, or temporarily or completely stop your treatment with PADCEV if you get severe side effects.
If your healthcare provider prescribes PADCEV in combination with pembrolizumab for you, also read
the Medication Guide that comes with pembrolizumab for important information about pembrolizumab.
If your healthcare provider prescribes PADCEV in combination with the medicines pembrolizumab or pembrolizumab and berahyaluronidase alfa-pmph, also read the Medication Guide that comes with these medicines for additional important information.
The most common side effects of PADCEV when used in combination with pembrolizumab include:
•
Changes in liver function and kidney function tests
•
Rash. See “What is the most important information I should know about PADCEV?”
•
Increased sugar (glucose) in the blood. See “High blood sugar (hyperglycemia)”
•
Numbness or tingling in your hands or feet. See “Nerve problems”
•
Increased lipase (a test done to check your pancreas)
•
Decreased white blood cell, red blood cell, and platelet counts
•
Tiredness
•
Decreased sodium, phosphate, and protein (albumin) in the blood
•
Itching
•
Diarrhea
•
Hair loss
•
Decreased weight
•
Decreased appetite
•
Increased uric acid in the blood
•
Increased or decreased potassium
•
Dry eye. See “Eye problems”
•
Nausea
•
Constipation
•
Change in sense of taste
•
Urinary tract infection
The most common side effects of PADCEV when used in combination with pembrolizumab include:
The most common side effects of PADCEV when used alone include:
•
Increased sugar (glucose) in the blood. See “High blood sugar (hyperglycemia)”
•
Changes in liver and kidney function tests
•
Decreased white blood cell, red blood cell, and platelet counts
•
Rash. See “What is the most important information I should know about PADCEV?”
•
Tiredness
•
Numbness or tingling in your hands or feet. See “Nerve problems”
•
Decreased protein (albumin), sodium, and phosphate in the blood
•
Hair loss
•
Decreased appetite
•
Diarrhea
•
Nausea
•
Itching
•
Increased uric acid in the blood
•
Dry eye. See “Eye problems”
•
Change in sense of taste
•
Constipation
•
Increased lipase (a blood test done to check your pancreas)
•
Decreased weight
•
Stomach (abdominal) pain
•
Dry skin
•
Changes in liver function and kidney function tests
•
Rash
•
Increased sugar (glucose) in the blood
•
Numbness or tingling in your hands or feet
•
Increased lipase (a test done to check your pancreas)
•
Decreased white blood cell, red blood cell, and platelet counts
•
Tiredness
•
Decreased sodium, phosphate, and protein (albumin) in the blood
•
Itching
•
Diarrhea
•
Hair loss
•
Decreased weight
•
Decreased appetite
•
Increased uric acid in the blood
•
Increased or decreased potassium
•
Dry eye
•
Nausea
•
Constipation
•
Change in sense of taste
•
Urinary tract infection
The most common side effects of PADCEV when used alone include:
•
Increased sugar (glucose) in the blood
•
Changes in liver and kidney function tests
•
Decreased white blood cell, red blood cell, and platelet counts
•
Rash
•
Tiredness
•
Numbness or tingling in your hands or feet
•
Decreased protein (albumin), sodium, and phosphate in the blood
•
Hair loss
•
Decreased appetite
•
Diarrhea
•
Nausea
•
Itching
•
Increased uric acid in the blood
•
Dry eye
•
Change in sense of taste
•
Constipation
•
Increased lipase (a blood test done to check your pancreas)
•
Decreased weight
•
Stomach (abdominal) pain
•
Dry skin
PADCEV may cause fertility problems in females and males, which may affect the ability to have children.
Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of PADCEV.

Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
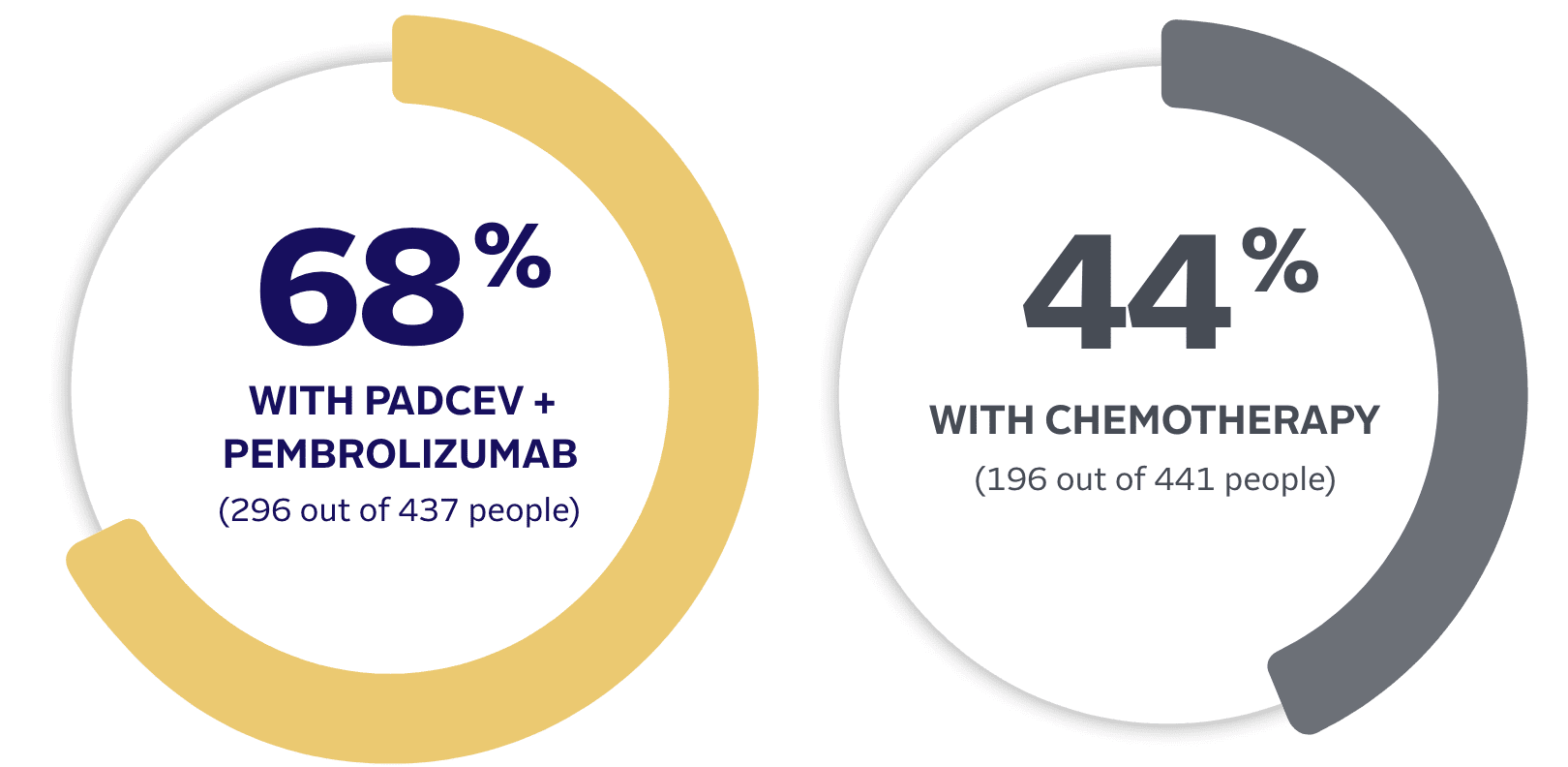
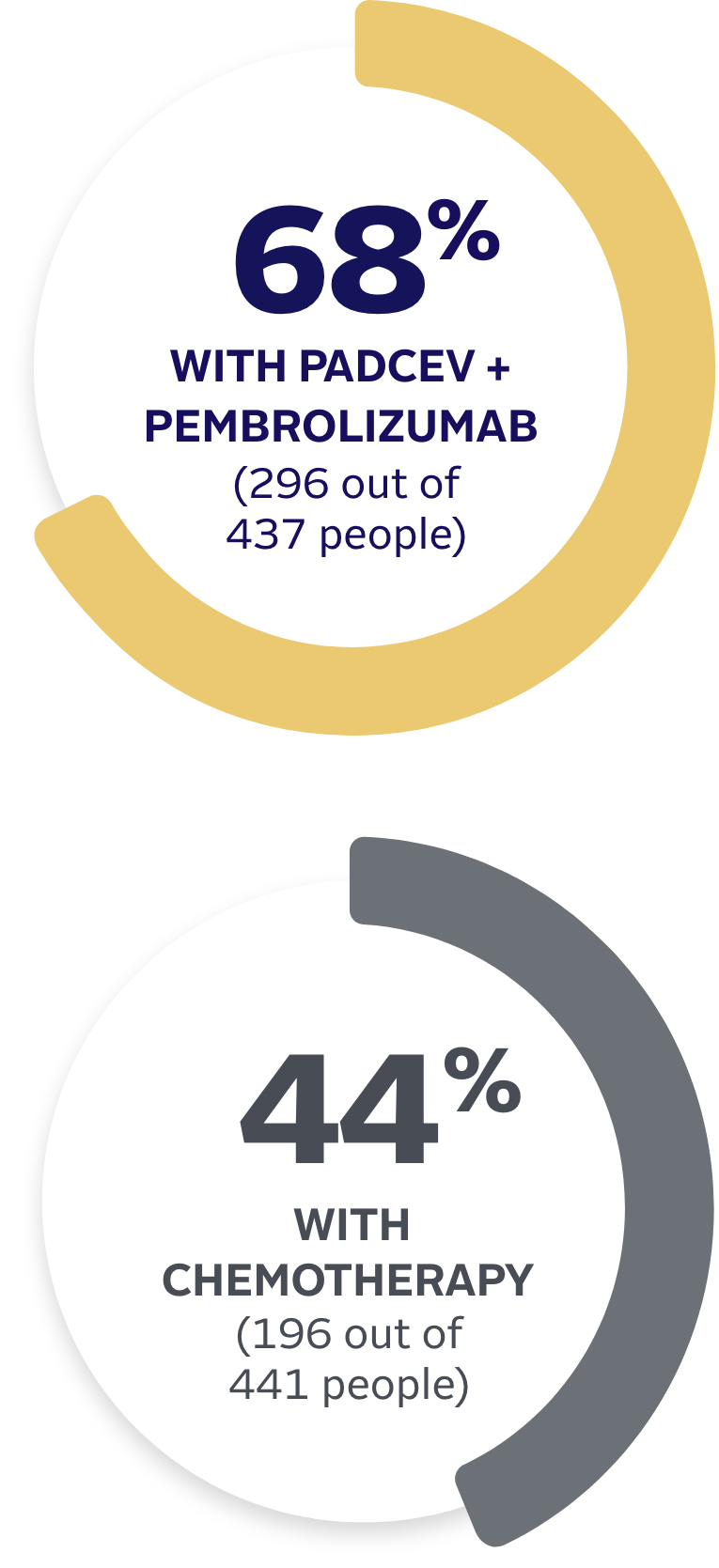
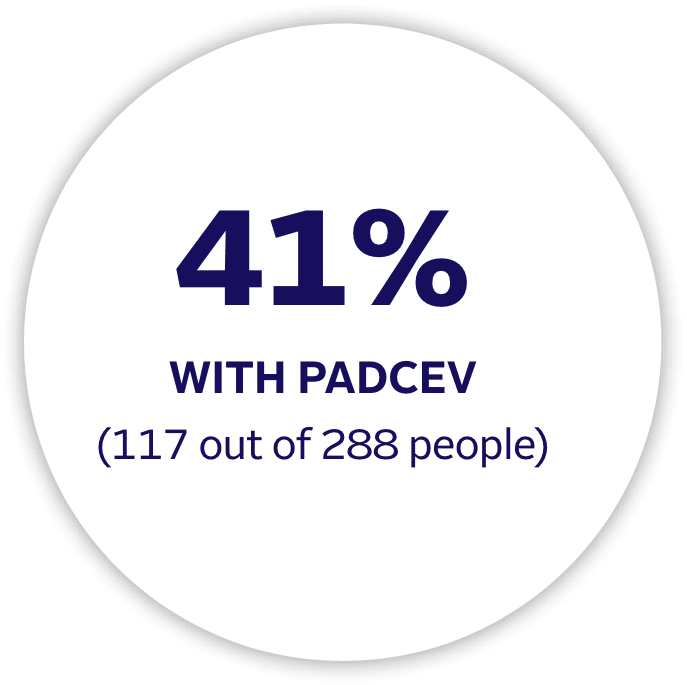
PADCEV is a prescription medicine used to treat adults with bladder cancer and cancers of the urinary tract (renal pelvis, ureter, or urethra).
PADCEV is a prescription medicine used to treat adults with bladder cancer and cancers of the urinary tract (renal pelvis, ureter or urethra) that has spread or cannot be removed by surgery.
•
PADCEV may be used with pembrolizumab or pembrolizumab and berahyaluronidase alfa-pmph before and after the surgical removal of your bladder when:
–your bladder cancer has spread into the muscle layer of the bladder (muscle invasive bladder cancer [MIBC]) but not to other parts of the body, and
–you are not able to receive chemotherapy that contains the medicine cisplatin.
•
PADCEV may be used with pembrolizumab or pembrolizumab and berahyaluronidase alfa-pmph when your bladder or urinary tract cancer has spread or cannot be removed by surgery (locally advanced or metastatic).
•
PADCEV may be used alone when your bladder or urinary tract cancer has spread or cannot be removed by surgery (locally advanced or metastatic) if you:
•
PADCEV may be used with pembrolizumab, or
•
PADCEV may be used alone if you:
–have received an immunotherapy medicine and chemotherapy that contains platinum, or
–are not able to receive a chemotherapy that contains the medicine cisplatin and you have received 1 or more prior therapy.
–have received PD-1 or PD‑L1 immunotherapy medicine and chemotherapy that contains platinum, or
–are not able to receive a chemotherapy that contains cisplatin and you have received 1 or more prior therapies.
It is not known if PADCEV is safe and effective in children.